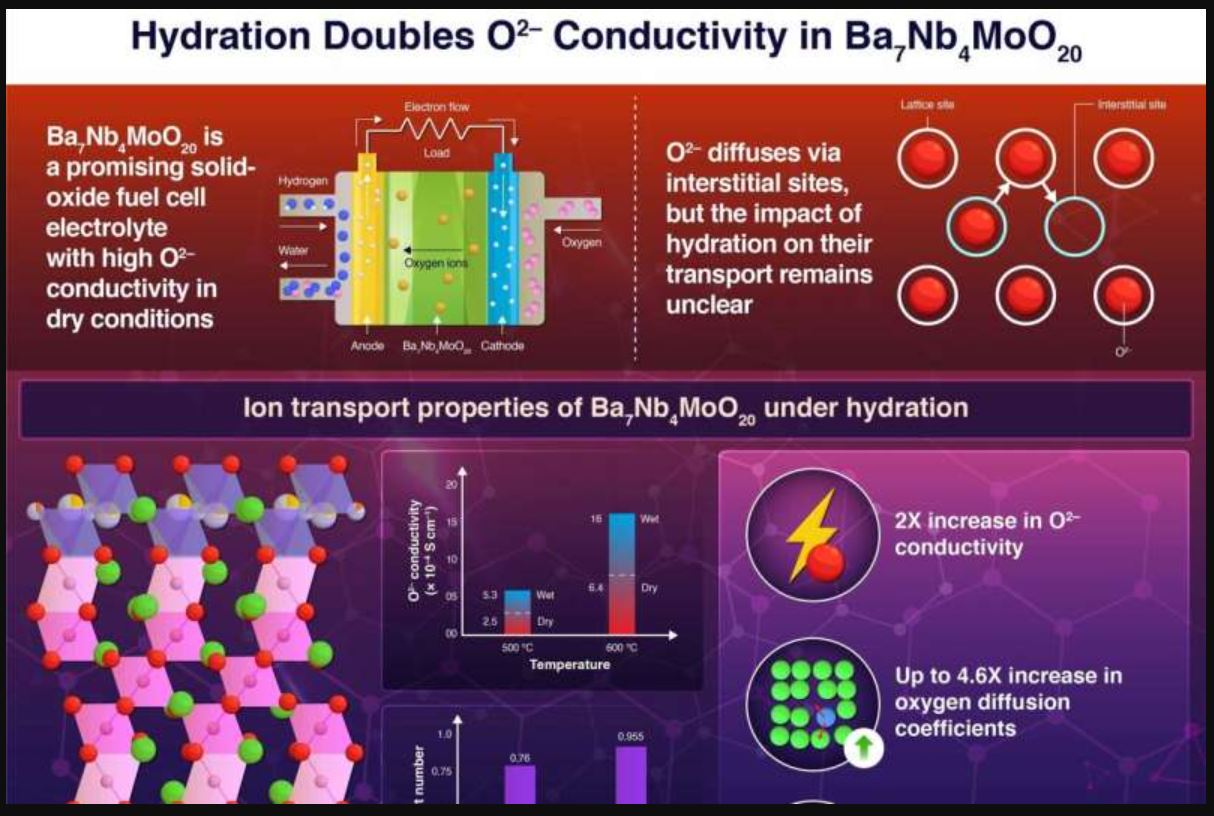
 Researchers at the Institute of Science Tokyo have uncovered a clever way to double the oxide-ion conductivity in the ceramic electrolyte Ba7Nb4MoO20—simply by using water vapor at 500 °C. This advance paves the way for more efficient and longer-lasting low-temperature solid oxide fuel cells, an exciting prospect for cleaner energy.
Researchers at the Institute of Science Tokyo have uncovered a clever way to double the oxide-ion conductivity in the ceramic electrolyte Ba7Nb4MoO20—simply by using water vapor at 500 °C. This advance paves the way for more efficient and longer-lasting low-temperature solid oxide fuel cells, an exciting prospect for cleaner energy.
Fuel cells, which generate electricity by combining hydrogen and oxygen, offer a promising low-emission energy solution. Traditionally, high operating temperatures of up to 1,000 °C have spurred rapid material degradation, a challenge anyone working with these systems can appreciate. Lowering these temperatures without sacrificing performance is key, and this research takes a big step in that direction.
The study revealed that hydration increases the number of interstitial oxygen ions in the material, thereby boosting oxide-ion mobility. To arrive at these findings, the team synthesised Ba7Nb4MoO20 pellets and compared their transport properties under both dry and humid conditions. They found that in humid air, the material’s conductivity more than doubled, with O2- ions acting as the primary charge carriers.
Further support came from molecular dynamics simulations, which showed that water vapor assists in forming (Nb/Mo)2O9 dimers within the crystal lattice—a structural change that makes it easier for oxide ions to move. If you’ve ever wrestled with the challenges of material degradation at high temperatures, you’ll appreciate how this discovery could simplify design issues in fuel cells.
This research not only fills a critical gap in our understanding of interstitial oxygen conductors but also opens the door to more efficient solid oxide fuel cells that operate at lower, more manageable temperatures. Such advancements could have a meaningful impact on clean energy tech and help us advance sustainable development worldwide.