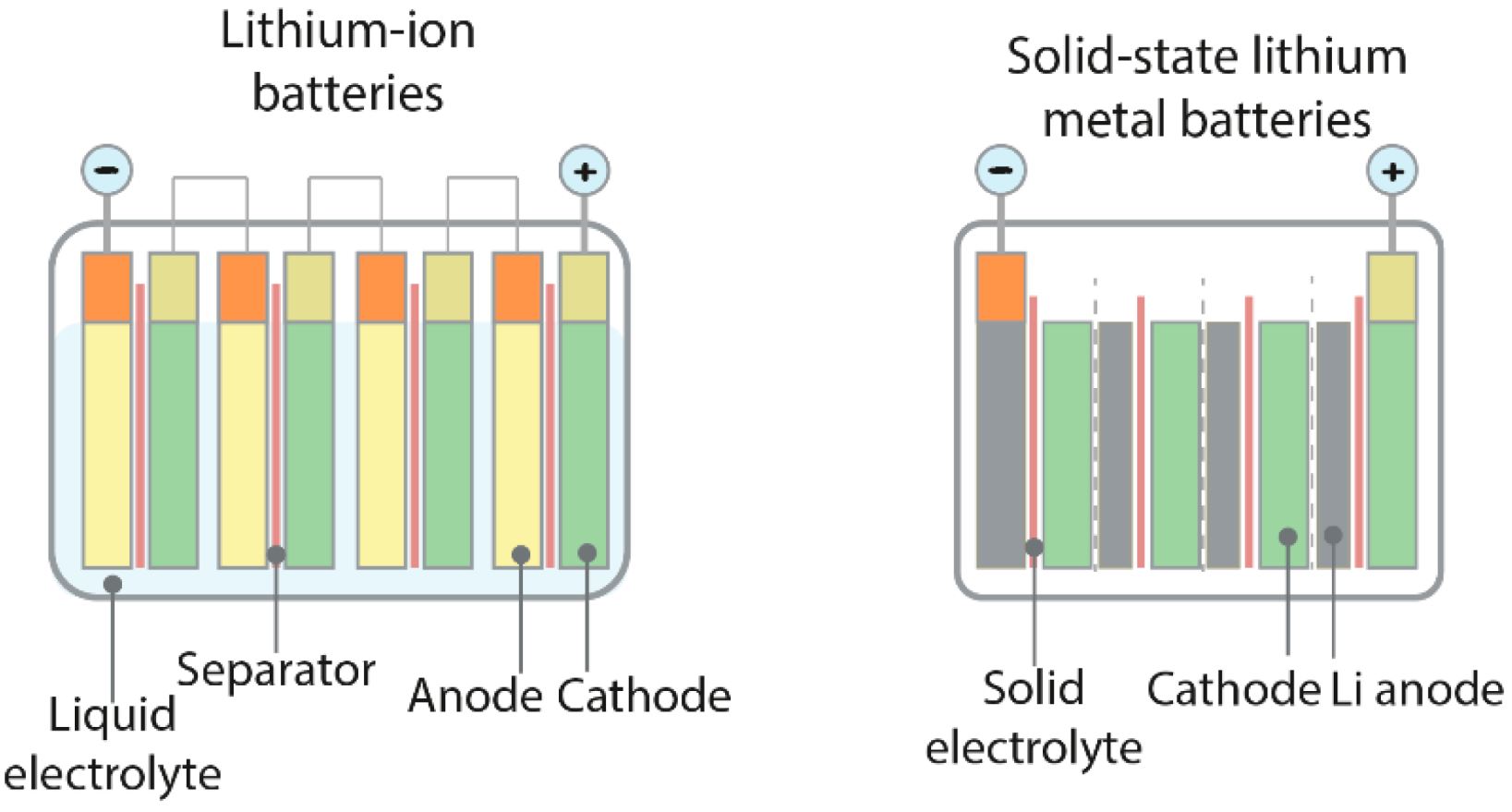
 Battery technology is constantly evolving, and researchers are making great strides in improving how we power our devices. If you’ve been following the developments in this field, you might be excited to hear about the latest advancements in lithium-metal batteries (LMBs). These batteries, which use a lithium-metal anode, promise higher energy storage compared to the more common lithium-ion batteries (LiBs). This is great news for electric vehicles and high-tech gadgets that demand more power.
Battery technology is constantly evolving, and researchers are making great strides in improving how we power our devices. If you’ve been following the developments in this field, you might be excited to hear about the latest advancements in lithium-metal batteries (LMBs). These batteries, which use a lithium-metal anode, promise higher energy storage compared to the more common lithium-ion batteries (LiBs). This is great news for electric vehicles and high-tech gadgets that demand more power.
However, there’s been a bit of a hitch. While LMBs offer more energy, they’ve struggled with issues like stability, safety, and slow charging speeds, which have kept them from becoming mainstream. But here’s where it gets interesting. Researchers from the Korea Advanced Institute of Science and Technology (KAIST) and their partners have made a breakthrough. They’ve developed innovative electrolytes using symmetric organic salts that significantly boost the performance of LMBs. This exciting research was published in Nature Energy.
So, what’s the secret sauce here? These new electrolytes prevent the formation of dendrites, which are pesky little structures that can degrade battery performance over time. As Akito Sakai, Yosuke Matsumoto, and their team explain, “The realization of LMBs requires an electrolyte that combines non-flammability with high electrochemical stability.” While current technologies have improved LMB life cycles, creating an electrolyte that balances high performance and safety has been a tough nut to crack.
To tackle this, the researchers incorporated an ionic plastic crystal called 1,1-diethylpyrrolidinium bis(fluorosulfonyl)imide (Pyr2(2)FSI) into traditional electrolytes. This change modifies how lithium ions move within the battery, making them more agile. “We created miniature anion–Li+ solvation structures by introducing symmetric organic salts into various electrolyte solvents,” the team noted. These structures allow for high ionic conductivity and low desolvation barriers, which are crucial for better battery performance.
By lowering the desolvation barrier, these new electrolytes make it easier for lithium ions to reach the electrodes, speeding up charging times and extending the battery’s lifespan. They also help form a stable protective layer on the lithium-metal anode, which prevents degradation over time.
In tests, these electrolytes showed remarkable improvements in LMB stability and cycling speed. Their non-flammable nature and resistance to overheating make them safer for real-world applications. “Our electrolyte design enables stable, fast cycling of practical LMBs with high stability (LiNi0.8Co0.1Mn0.1O2 cell (twice-excessed Li): 400 cycles) and high power density (pouch cell: 639.5 W kg−1),” the researchers stated. Impressively, the Li-metal pouch cell even survived a nail penetration test, showcasing its high safety.
This development is a promising leap toward making lithium-metal batteries a viable, safe, and efficient power source for a wide range of applications. It’s an exciting time for battery technology, and these advancements could soon change how we power our world.








