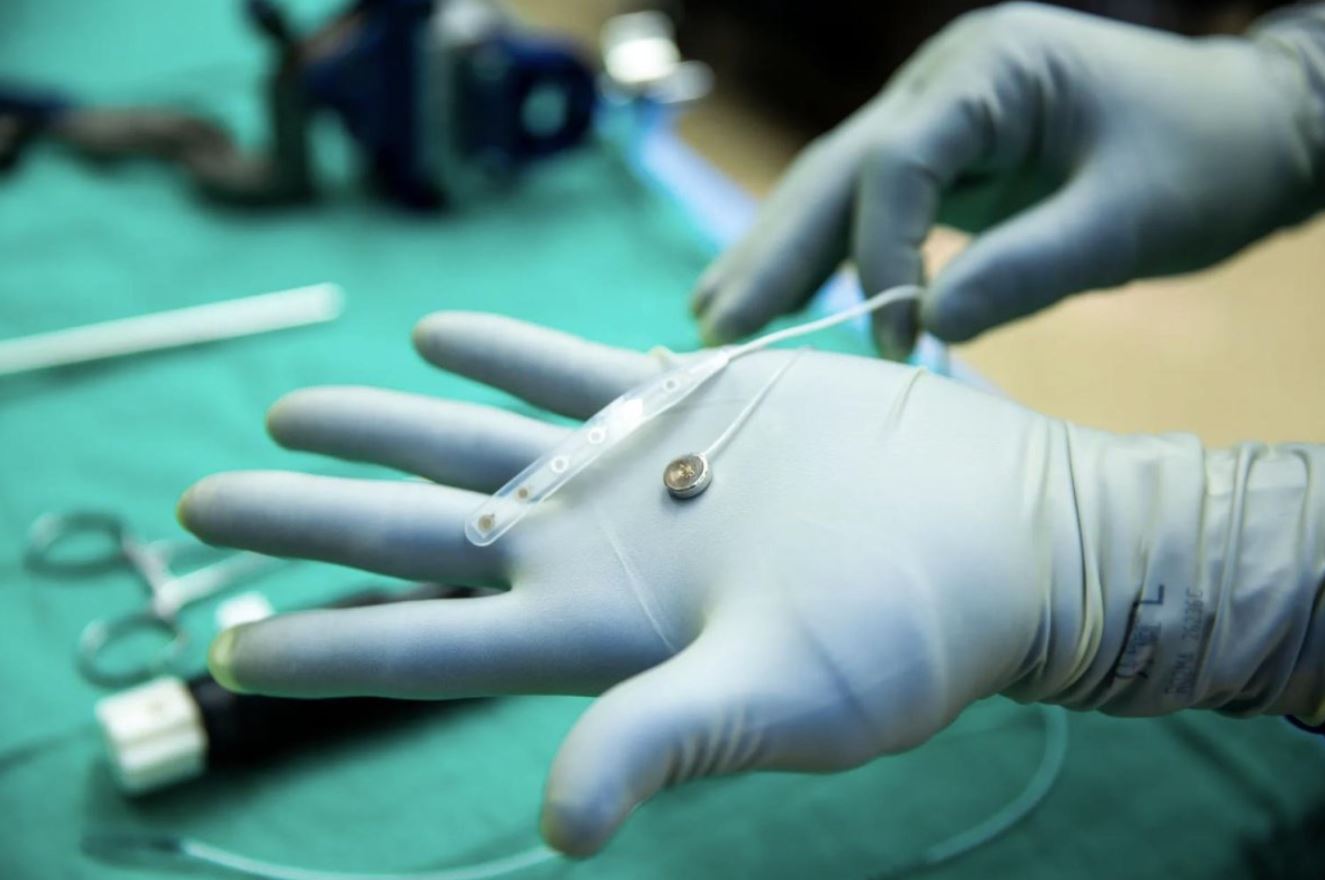
 Paradromics has just reached a milestone by implanting its brain-computer interface (BCI) device in a human during a routine epilepsy surgery at the University of Michigan. The entire process took only 20 minutes, highlighting the potential of their Connexus system, which reads neural signals at the neuron level. While the device isn’t FDA-approved yet, this breakthrough offers a promising glimpse into the future of clinical trials.
Paradromics has just reached a milestone by implanting its brain-computer interface (BCI) device in a human during a routine epilepsy surgery at the University of Michigan. The entire process took only 20 minutes, highlighting the potential of their Connexus system, which reads neural signals at the neuron level. While the device isn’t FDA-approved yet, this breakthrough offers a promising glimpse into the future of clinical trials.
The Connexus was put to the test in a carefully controlled environment, as doctors managed the procedure during routine brain surgery. CEO Matt Angle summed it up by saying, “This surgery is a pivotal moment for Paradromics.” His words reflect the company’s readiness to transition from preclinical success to potential patient benefits, especially for those facing severe motor impairments such as paralysis.
In a field growing ever more competitive—with notable players like Elon Musk’s Neuralink, as well as Synchron and Precision Neuroscience—Paradromics is poised to make a real difference. Their mission is simple: help individuals regain communication abilities by harnessing sophisticated BCI technology. The fact that the trial was performed under controlled conditions ensures that patient safety was never compromised.
With nearly $100 million raised and a global partnership with Saudi Arabia’s NEOM project, Paradromics is not just thinking locally but on a worldwide scale. The company is gearing up for full-scale clinical trials later this year to thoroughly evaluate safety and long-term benefits for those living with disabilities.








